Regulatory Shorts#3 |Data Lock Point, International Birth Date, Development International Birth Date - YouTube

Authoring a periodic adverse drug experience report…here's what you need to know! Kulkarni TN, Kulkarni NG - Perspect Clin Res

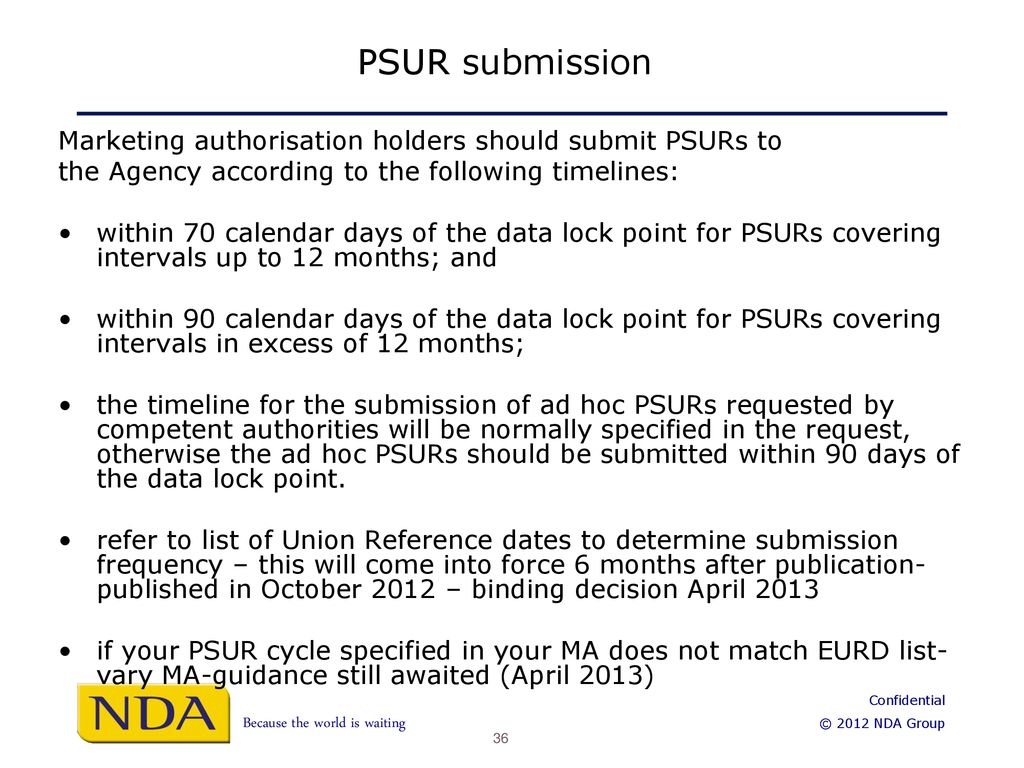
Dr. Shripadaraja.R on Twitter: "What are the timelines for the submission of PSURs? #drugsafety #pharmacovigilance #clinicaltrials #drugdevelopment #pharmcompanies #clinicalresearch #MAH #CRO #BPO #healthcare #lifesciences #research #biotech #vaccines ...

Authoring a periodic adverse drug experience report…here's what you need to know! Kulkarni TN, Kulkarni NG - Perspect Clin Res

The Pharmacovigilance Medical Writer: Medical Writer, Project Manager, Regulatory Expert - Trilogy Writing & Consulting GmbH