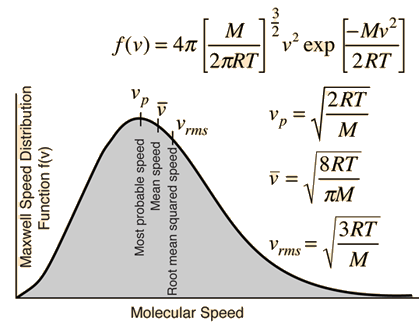
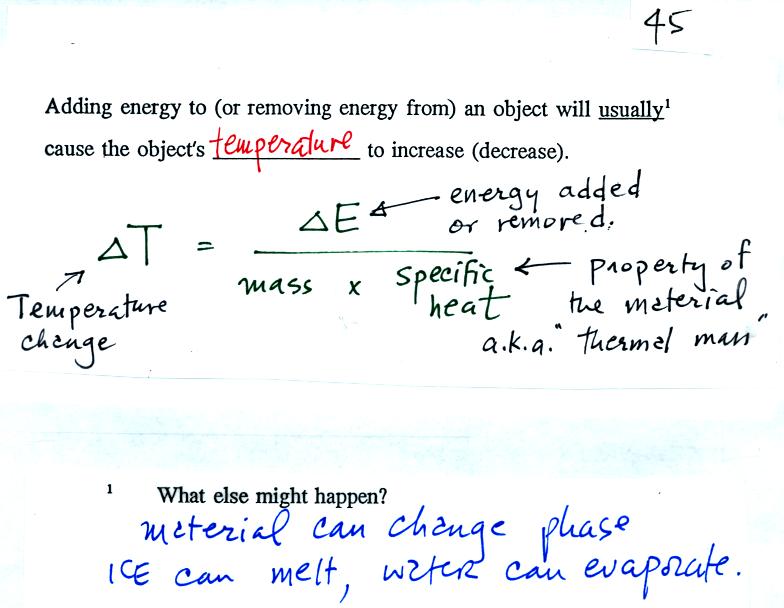
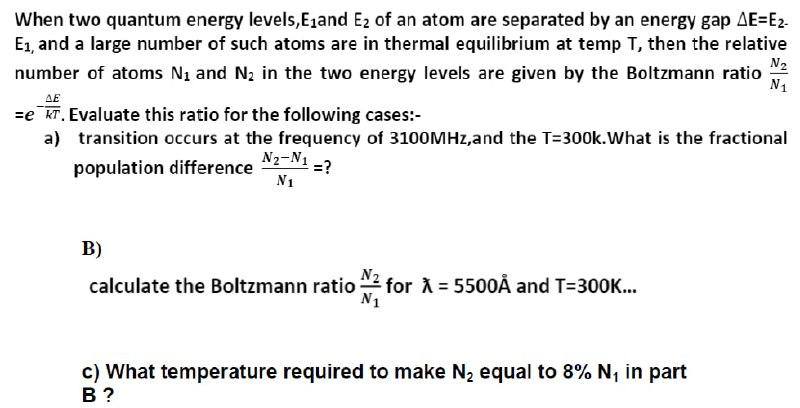
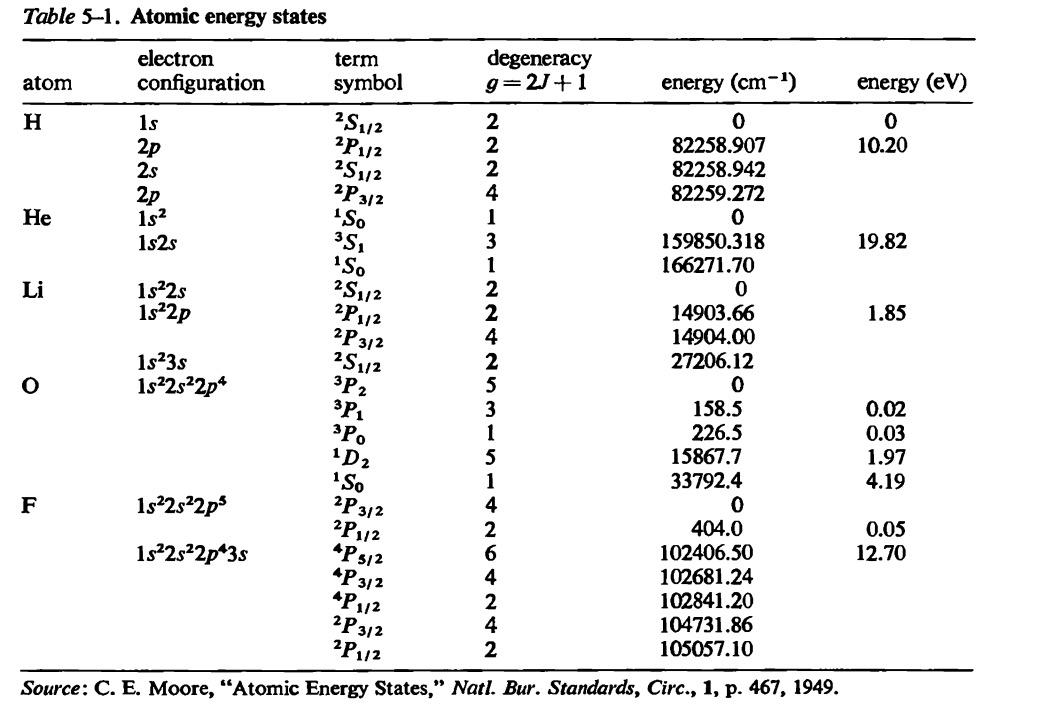
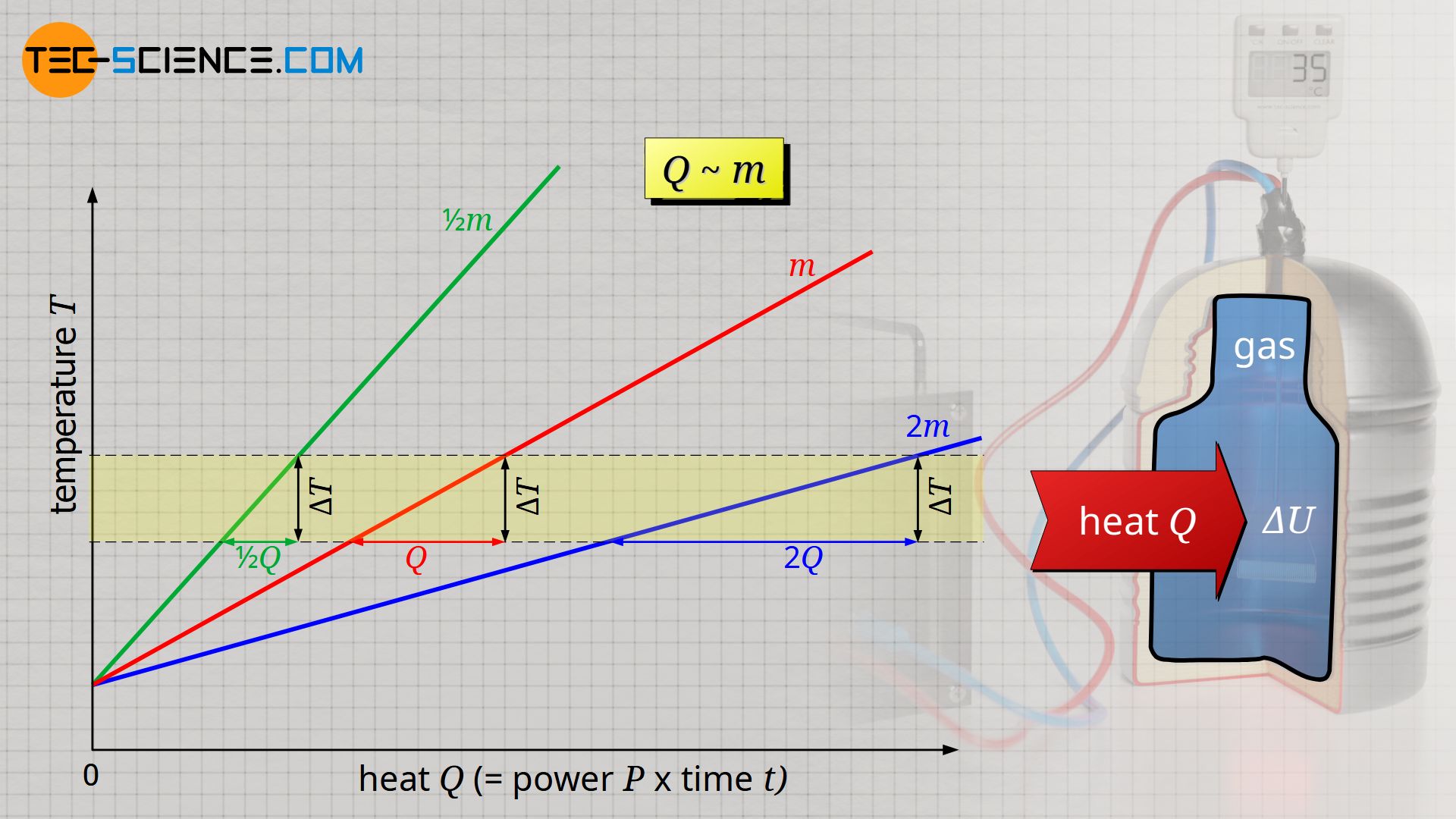
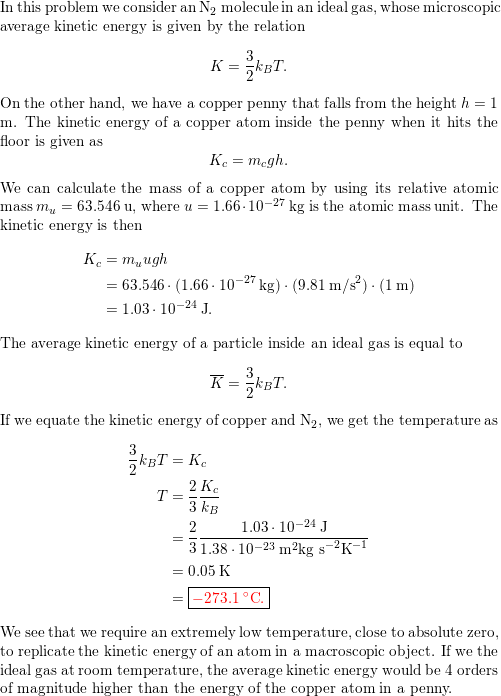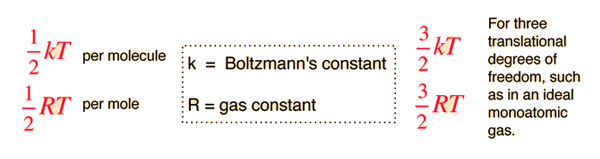SOLVED:In Part IV you'll learn to calculate that 1 mole (6.02 ×10^23 atoms) of helium atoms in the gas phase has 3700 J of microscopic kinetic energy at room temperature. If we
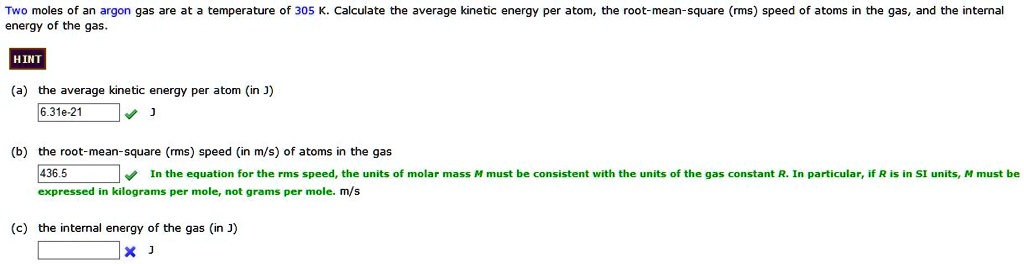
SOLVED: Two moles an argon gas are energy of the gas temperature of 305 K. Calculate the average kinetic energy per atom Toot mean square (rms) speed atoms in the gas, and

Thermal Energy Equation & Examples | How to Calculate Thermal Energy - Video & Lesson Transcript | Study.com

Total energy per atom as a function of temperature. Kinetic energy,... | Download Scientific Diagram
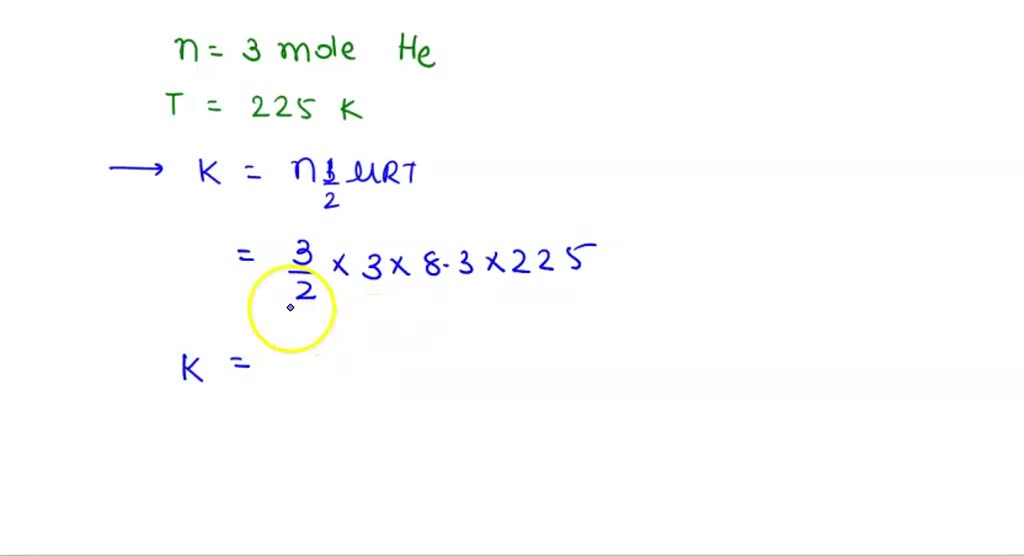
SOLVED: Five moles of a helium gas are at a temperature of 245 K. Calculate the average kinetic energy per atom, the root-mean-square (rms) speed of atoms in the gas, and the

Calculate the increase in energy (in joule) per atom of a piece of aluminium when its temperature is raised by 1^(@)C. Given 27 g of aluminium contains 6xx10^(23) atoms, and specific heat

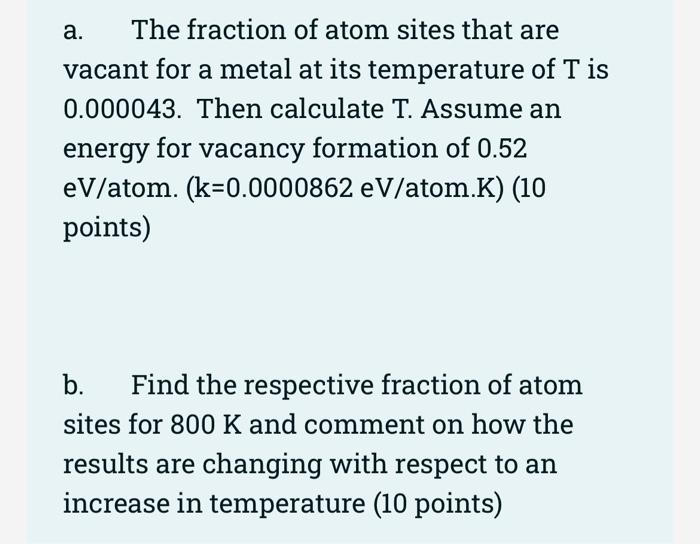
SOLVED: Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327*C. Assume an energy for vacancy formation of 0.52 eVlatom: When, Boltznann constant K =

SOLVED:Calculate the fraction of atom sites that are vacant for copper at its melting temperature of 1084^∘ C(1357 K) . Assume an energy for vacancy formation of 0.90 eV / atom.