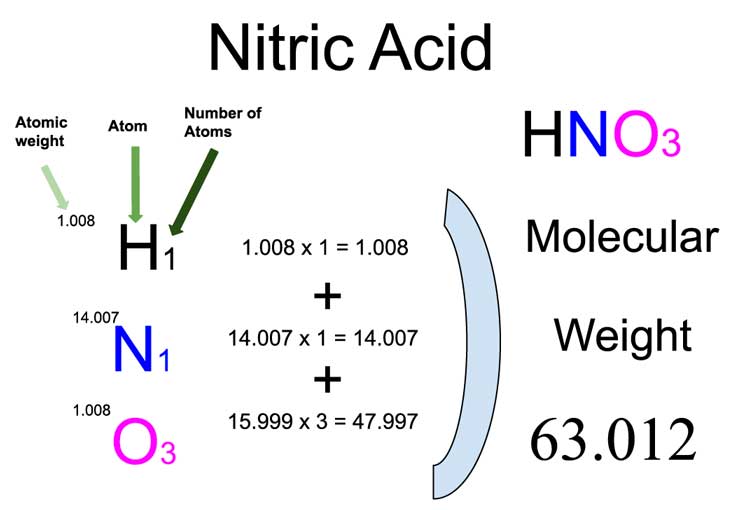
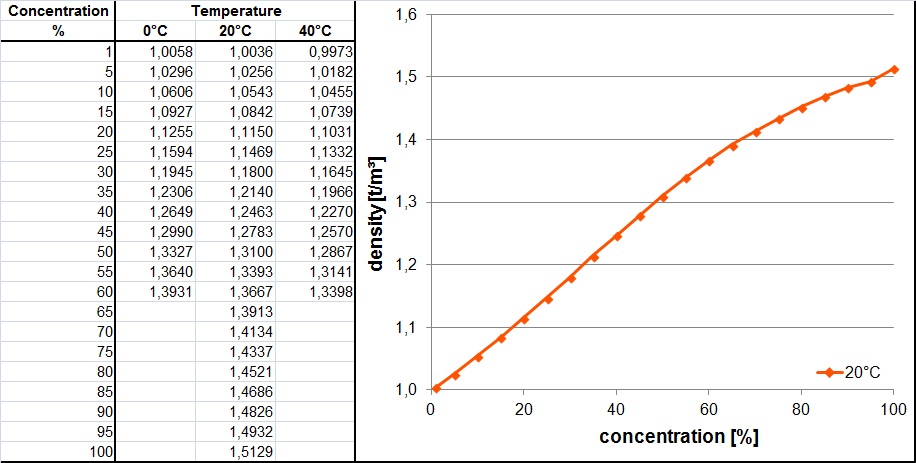
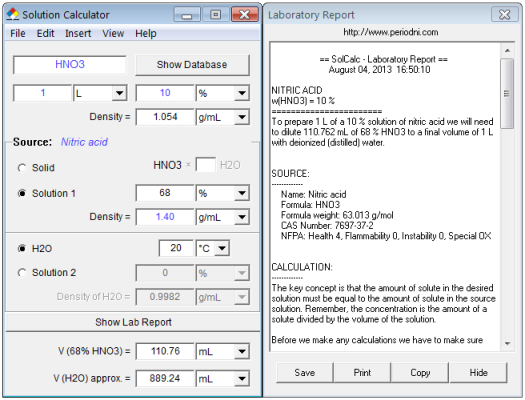
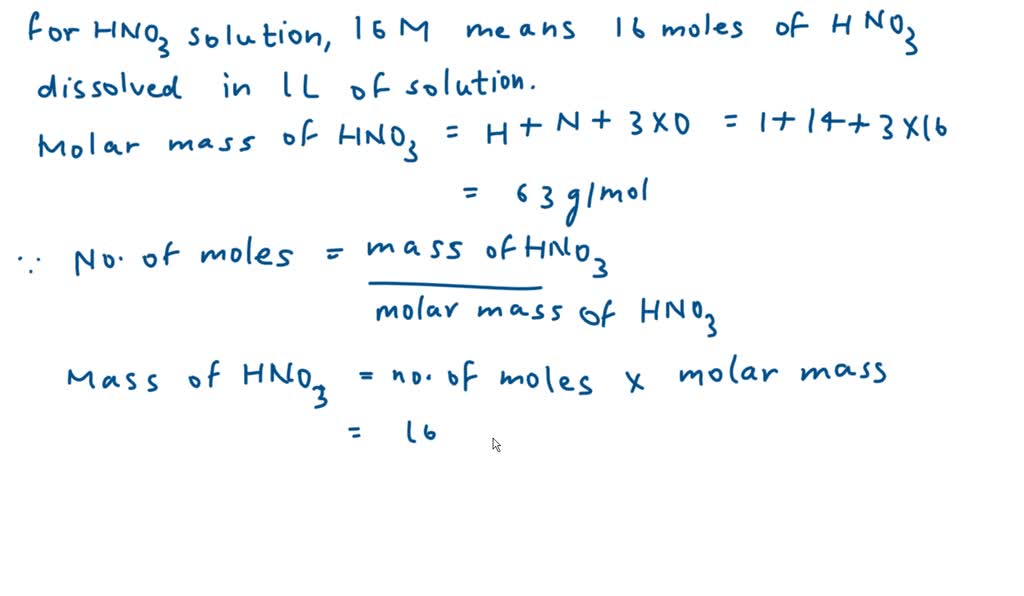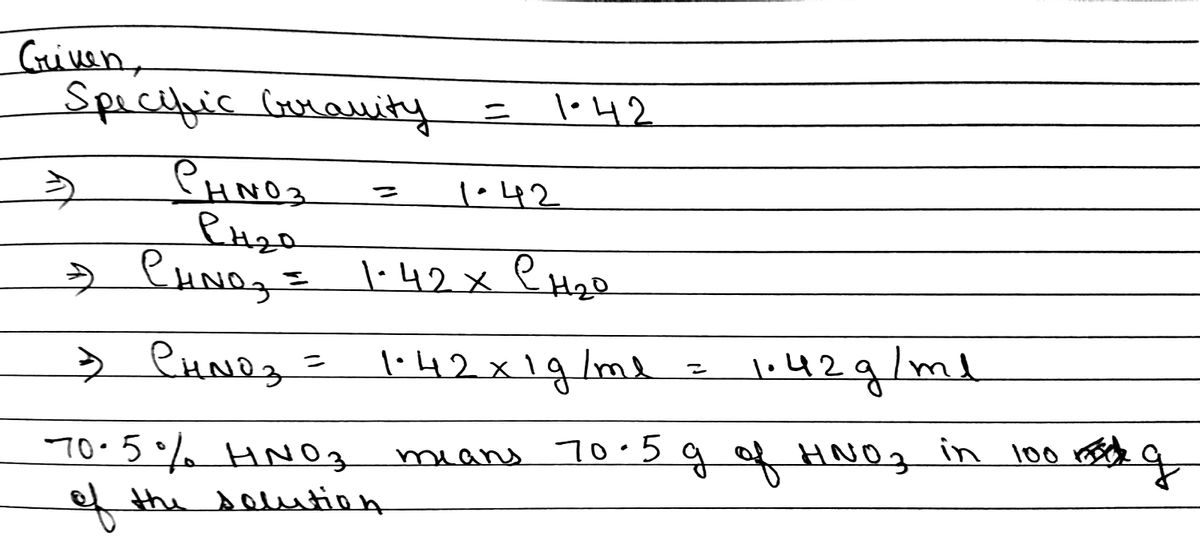Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

SOLVED: HNO3 has a concentration of 0.003 M. Write the dissociation reaction of the acid and then, WITHOUT using a calculator, determine the pH range of this stock solution.

Calculate the concentration of nitric acid in moles per litre in a sample which has a density - YouTube

Question Video: Calculating the Concentration of Nitric Acid via Titrating against a Known Volume of Potassium Hydroxide | Nagwa

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g //mL and the mass percent of nitric acid in it being 69%.
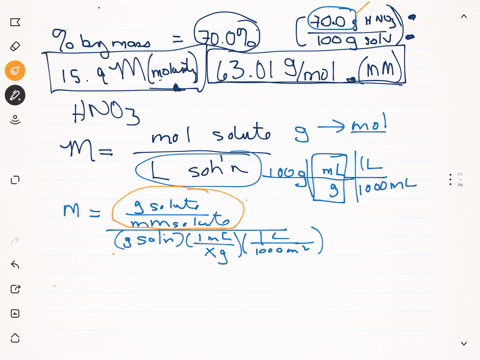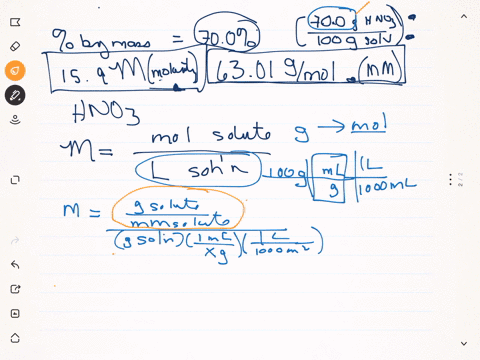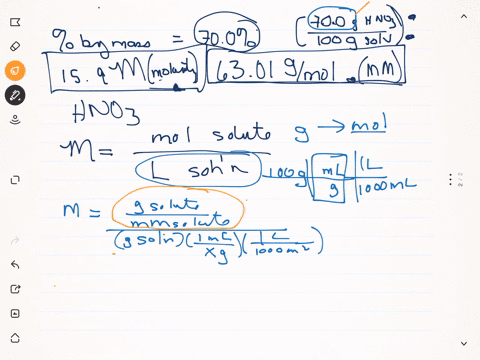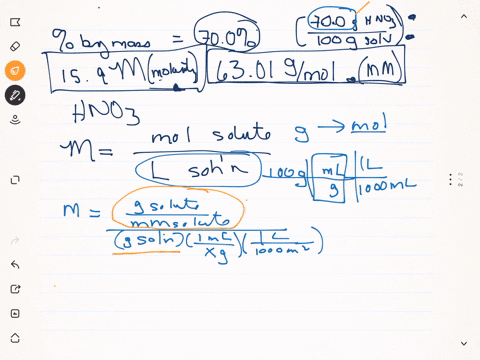
SOLVED: Q22: Calculate the molar concentration of HNO3 (63.0 g/mol) in a solution that has a density of 1.42Kg/L and is 70.5% HNO3 (w/w) (Answer ~ 16M)