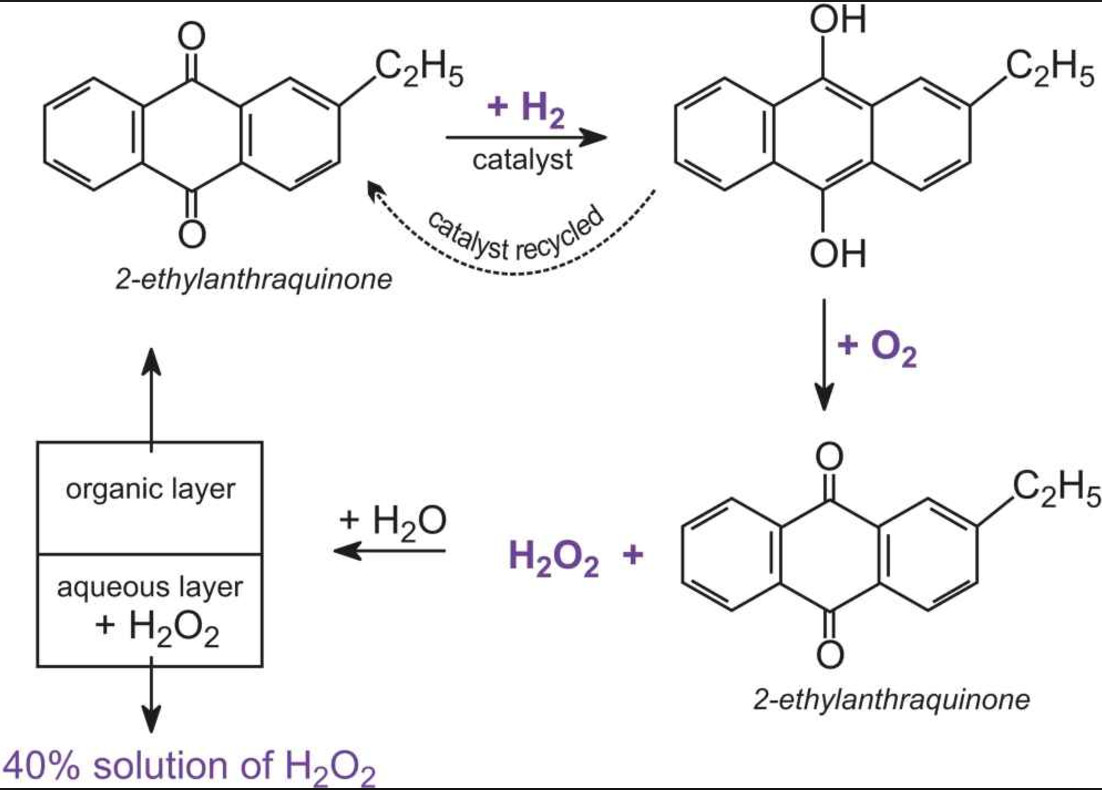
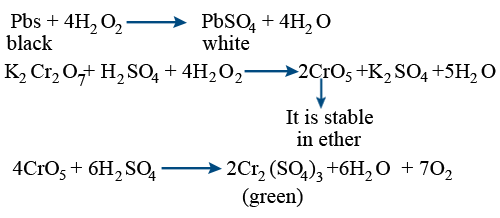
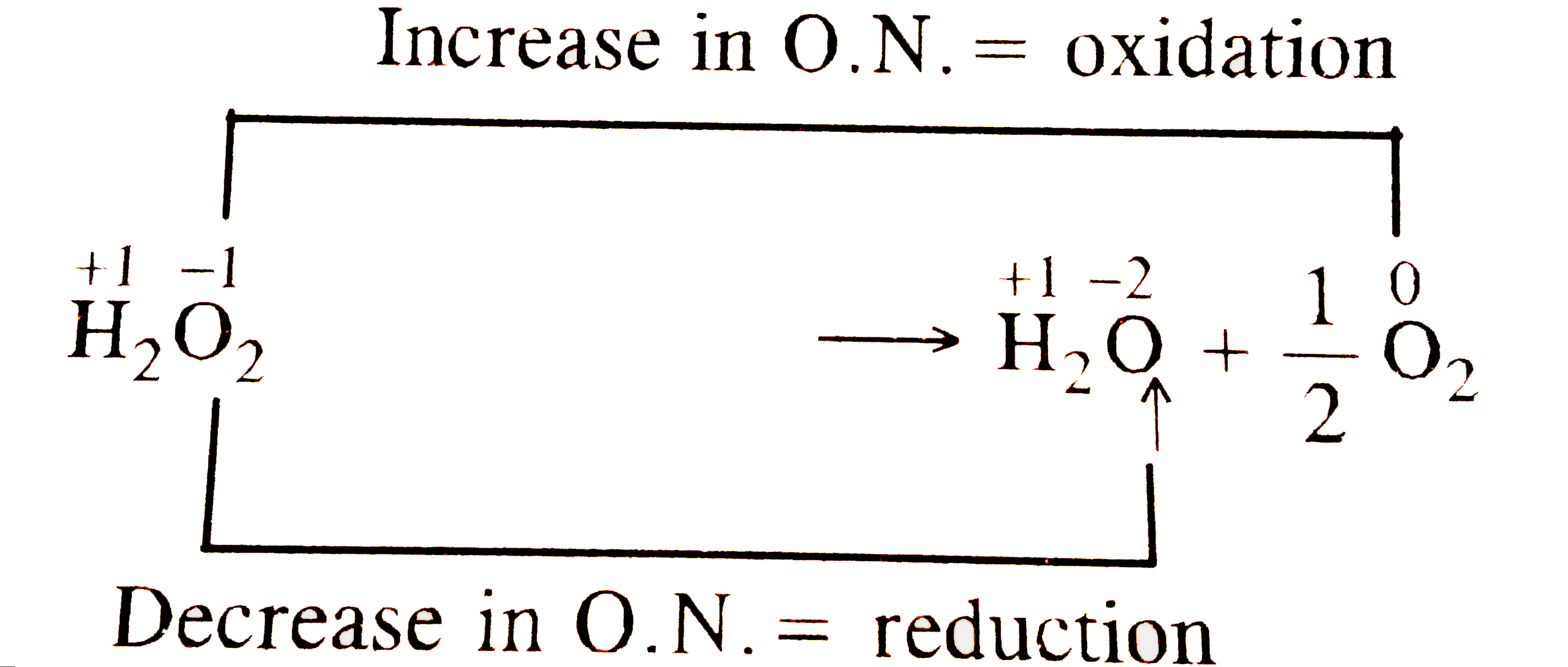How will you show that H2O2 acts as both oxidising and reducing agent? What is meant by '30 volume' of H2O2? - Quora

While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why ?

An acidic solution of hydrogen peroxide behaves as an oxidizing as well as reducing agent. Illustrate it with the help of a chemical equation.
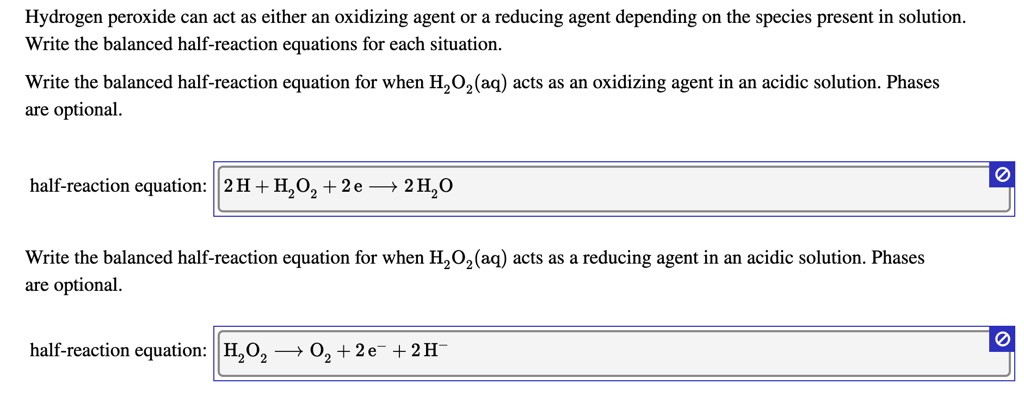
SOLVED: Hydrogen peroxide can act as either an oxidizing agent Or a reducing agent depending on the species present in solution Write the balanced half-reaction equations for each situation Write the balanced

An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. - YouTube

how can hydrogen peroxide be both oxidising and reducing agent - Chemistry - Redox Reactions - 13207969 | Meritnation.com
Hydrogen peroxide acts as both a reducing agent and oxidizing agent depending upon the nature of the reacting species. In which case does peroxide act as a reducing agent in acid medium? -

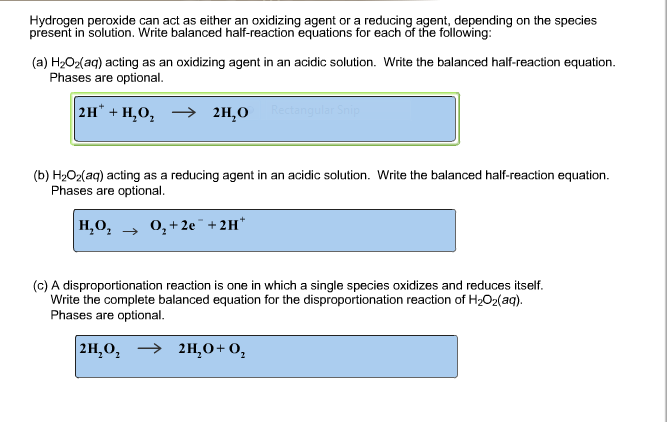
Hydrogen peroxide can act as either an oxidizing agent or a reducing agent, depending on the species present in solution. Write balanced half-reaction equations for each of the following: (a) H2O2(aq)

Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases H(2)O(2) acts as a reducing agent
While sulphur dioxide and hydrogen peroxide can actas oxidising as well as reducing agents in theirreactions, ozone and nitric acid act only as oxidants.This is because(a) in SO2 and H2O2, S and

While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their - Brainly.in
Show how H2O2 functions both as a reducing agent and as a oxidizing agent? - Sarthaks eConnect | Largest Online Education Community